Previous Projects
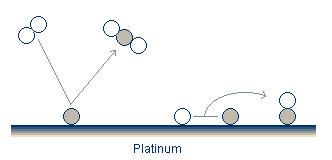
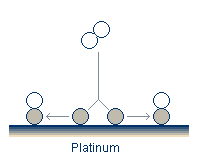
All models for the reaction-rate oscillations at atmospheric pressure share several common features. Oxygen adsorbs and dissociates in a pair of free adsorption sites. Carbon monoxide also adsorbs onto the catalyst surface.
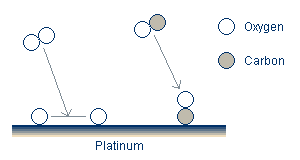
In addition to the adsorption, the reactants can desorb, although the desorption of oxygen is often neglected as it is believed to be quite slow. Finally, the activation energy required to form the CO2 is greatly reduced on the surface compared to its value in the gas stream. The reaction therefore takes place on the surface, and the newly formed CO2 rapidly desorbs.
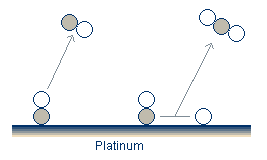
In the oxidation-reduction model,1 there is, in addition to the adsorbed O and CO, a more tightly bound subsurface oxide. The rate at which this oxide forms and is removed from the platinum surface is much slower than the other processes occurring in the system. As a result it is the oxide state which controls the oscillations.
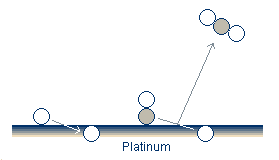
We have made a wide variety of comparisons to this model and have found a good qualitative agreement in nearly all cases. As such, the oxidation-reduction model is the leading candidate to explain the oscillations which we have observed.
The carbon model2 proposes adsorbed atomic carbon impurities as the controlling species on the platinum surface. The main drawback to this model is its inability to explain the source of the carbon. Suggestions include diffusion from the bulk platinum of from the reactant gases.
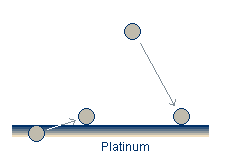
The carbon impurities can be removed through a number of mechanisms including reaction with gaseous oxygen to form CO or CO2 molecules or reaction with adsorbed oxygen to form an adsorbed CO molecule.
| 1 |
"The oxidation and CO reduction kinetics of a platinum surface."
B. C. Sales, J. E. Turner, and M. B. Maple,
Surf. Sci. 112, 272-80 (1981).
|
| 2 | "Studies on self-sustained reaction-rate oscillations III: The carbon model." N.A. Collins, S. Sundaresan, and Y.J. Chabal, Surf. Sci. 180, 136-52 (1987). |