Previous Projects
This system has been designed to be spatially uniform on all parts of the catalyst. In order to study reaction-rate front propagation in a controlled manner, an experiment was conducted in which the spatial uniformity was intentionally broken by applying small amounts of CO to one part of the catalyst surface. Perturbations were applied to catalysts operating in both the steady-state and oscillatory regimes.
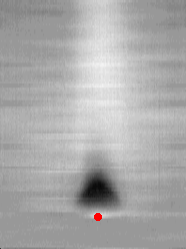
A small amount of CO was applied to the surface of a catalyst operating under steady-state conditions. The excess CO caused the local portion of the catalyst to make a transition to the lower reaction-rate branch. After a short time the excess CO was reacted and advected away from the catalyst and the catalyst returned to the steady state once again. In the figure below, the perturbation was applied at the place and time indicated by the red dot. The transition, shown in black, is limited in both space and time.
 | |
Having shown that the perturbations are limited in both space and time, a smaller perturbation was applied to the surface of an oscillating catalyst during the portion of the oscillatory cycle where the catalyst was on the upper reaction-rate branch. As shown in the figure below, when the perturbation was applied to the catalyst (at the time and location indicated by the red dot), it induced an oscillation across the entire catalyst. Examination of this oscillation showed that it is nearly identical to the naturally occuring oscillations.
 | |
The induced oscillations were not observed to affect the phase of the oscillatory cycle. This behavior remains unexplained because models of this system predict that the phase of the oscillations should shift to match the time the perturbation was applied.
|
| C. D. Lund, C. M. Surko, M. B. Maple, and S. Y. Yammoto, J. Chem. Phys. 108 (1998), pp. 5565-70. |