Previous Projects
Convection
From 1988 to 2005 we carried out experiments on convection in binary fluid mixtures. This research was done by Roman Sokolov, Christof Aegerter, Arthur La Porta, Keith Eaton, Dan Ohlson, Bill Baxter, Cliff Surko, and other collaborators.
CONVECTION IN BINARY FLUID MIXTURES
When a thin layer of fluid is confined between parallel plates and heated from below, the fluid becomes unstable to a flow consising of overturning convection rolls. This is known as Rayleigh-Benard convection. From a fluid-dynamics point of view, this flow enhances heat transport through the fluid layer. In this research group, we are more concerned with the fact that the onset of the flow spontaneously breaks the translational symmetry of the system. It is an example of a "nonequilibrium phase transition." We study it as an example of pattern formation in a system driven far from equilibrium.
In a pure fluid, the convection rolls overturn steadily and result in a nominally stationary pattern that (in some range of parameters) tends to evolve toward an ordered pattern. In a fluid mixture, the convection can be oscillatory, resulting in a traveling-wave pattern. Our research involves characterizing these very complicated patterns. Most of the previous work in traveling-wave convection was done using a restricted geometry. Our new apparatus allows us to study large aspect ratio 2D patterns, such as the one at the top of this page. Some topics of our research are described below.
Some topics of our research are described below.
Angular and curved textures in stationary convection patterns
Dynamics of traveling wave patterns
Spatiotemporal disorder in traveling-wave convection
Catalysis
Between 1995 and 2000 we did experiments on the heterogeneous catalysis process. This research was carried out by Chris Lund, Steve Yamamoto, Cliff Surko, and other collaborators. More information about this work
Under certain conditions of gas temperature and partial pressures, the rate at which carbon monoxide is oxidized to form CO2 oscillates in time. These reaction rate oscillations have been observed under both UHV and atmospheric pressures, but our interest is in understanding the mechanisms behind the oscillations and how they form spatial patterns at atmospheric pressure.
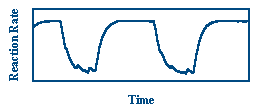
The formation of CO2 is an exothermic reaction, so observing changes in the temperature of the catalyst provides a measure of the rate of reaction. In this experiment, the temperature of the catalyst is measured in two ways. First, the temperature of the catalyst can be measured at all points simultaneously using infrared imaging.
The picture above shows the catalyst as it is seen by the IR imager. The quartz substrate is the gray ring in the center of the image and the platinum is the bright inner portion of the ring. Three supporting thermocouples are seen as triangles around the ring. In addition to IR imaging, thermocouples measure the local temperature of the catalyst near that thermocouple. This provides temporal information on the rate of reaction. The graph below shows oscillations in the temperature of a catalyst as measured by one of the thermocouples.
- Pulses which rotate around the ring
- Tests to determine the mechanisms behind spatial coupling in the system
- Local perturbations to break the spatial symmetry in a controlled manner
- Tests to discriminate between two models of the system